Hydrogen Generator
~ Ranjith, Pratap, Abilash
In Stem Land, we were doing Electrolysis experiment with water in order to explain the children how the electrolysis process works. The Materials that are we used to do this experiments are the following. Salt water, aluminium foil, copper wire, voltage source and two plastic glass. In this experiments water is the best method of producing high purity hydrogen gas. The most important element of the generator is the electrolysis cell where the electrolysis reaction takes place.
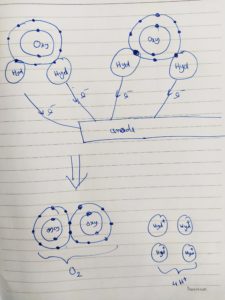
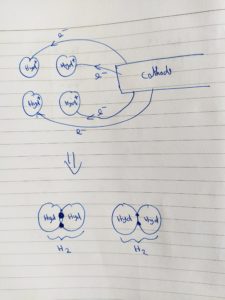
The cell consists of two electrodes (an anode and a cathode), which are separated by the ion exchange membrane (salt water). When a continuous 12 voltage is applied to the electrodes on the electrolyser cell, the following reaction happens:
Anode 2H2O – 4e = O2 + 4 H+
Cathode 4H+ + 4e = 2H2
But in-order to produce the highest purity of hydrogen up to up to 99.9995% purity, a platinum catalyst is used at the electrodes.
The students were very focused and eager doing the experiment and were coming up with different concepts.
Author
prathap7618@gmail.com
Related Posts

Bhishma Hunt program
The Bhishma Hunt program, organized by Sanvi Educational and Charitable Trust at Sanvi International School, invited STEM Land to lead robotics and...
Read out allPhysical and Mental Health Awareness Session
-SandhiyaBala, Sivaguru , Tamil, Durai, Ajay, Rajesh , Gunavathi, Poovizhi , SandhiyaSaravanan and Sri Bhavani Every Tuesday morning, we have a learning...
Read out allFractions using polypod
-Ajay Children from 6th grade at Isaiambalam School learned about proper, improper and mixed fractions through stories and visualizations using Polypad. This...
Read out all
RTL session for B.Voc students
~ Jayabharathy, Poonguzhali, Tamilarasan, Sandhiya Bala, Kugan, Duraisamy, Poovizhi RTL session was conducted for the B.Voc students on 14th and 21st of...
Read out allInspiring Young Minds at Our STEAM Camp
-Mathegramming Team A five day Science Technology Engineering Arts and Maths (STEAM) Camp was organised by all the Auroville schools from 27th...
Read out all
