Boyle’s law is a gas law that states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Boyle’s law was put forward by the Anglo-Irish chemist Robert Boyle in the year 1662.
For a gas, the relationship between volume and pressure (at constant mass and temperature) can be expressed as
P ∝ (1/V)
where P is the pressure exerted by the gas and V is the volume occupied by it. This proportionality can be converted into an equation by adding a constant, k.
P = k*(1/V) ⇒ PV = k
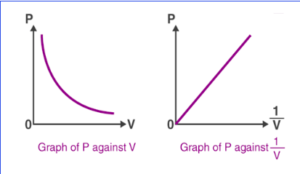
The pressure v/s volume curve for a fixed amount of gas kept at constant temperature is illustrated below.
Experiment using Thinktac kit:
This gas law describes how the pressure of a gas tends to increase as the volume of the container decreases. We use a syringe and balloon to understand the phenomenon.
Steps:
- Take a syringe of 60 ml capacity and remove its plunge.
- Take a small balloon.
- Blow a small amount of air into the balloon and hold at its neck tightly with your fingers.
- Insert the balloon through the syringe’s open end and remove it to check whether the balloon can enter freely inside the syringe or not.
- Now, tie a tight single knot at the balloon’s neck. Ensure that there is no air leakage.
- Insert the balloon into the syringe to the tip. Insert the plunger back to close the open end.
- Initially, push and set the plunger at the 30 ml reading on the syringe, with the tip of the syringe open. Now, tightly close the tip of the syringe with your finger.
- Press the plunger hard as much as possible and observe what happens to the balloon inside.
- After you reach the optimum level, release the plunger and continue to observe.
- Now, pull the plunger and set it at the 30 ml reading on the syringe again, with the tip of the syringe closed.
- Now, pull the plunger to the open end of the syringe and observe what happens to the balloon.
Filling water in the syringe:
- Take about 100 ml of water in a cut bottle container and the syringe with the balloon.
- Push the plunger till it reaches the balloon.
- Dip the tip of the syringe in the water and pull the plunger till the 60 ml reading fills the water.
- Hold the syringe with the syringe’s tip positioned upward as shown. Shake the syringe and adjust it to push air above the water level.
- Now, push the plunger slowly for the air above the water level to escape through the tip of the syringe.
- Push the plunger to remove the excess water into the container, till the plunger reaches the 40 ml reading on the syringe.
- Now, push and pull the plunger to the closed end of the syringe and observe what happens to the balloon.
Preparing the water balloon:
- Take another small balloon.
- Use the same 60 ml syringe and draw about 10 ml of water from the container.
- Empty the syringe before filling it with 10 ml of water.
- Now, insert the syringe’s tip into the mouth of the balloon and press the plunger to add water to the balloon’s neck.
- Pinch the balloon’s neck, at the point where the water is present, to prevent the air bubbles from entering the balloon.
- Now, twist the balloon’s neck, two times, and tie a knot tightly.
- Insert the balloon -filled with water into the syringe and close the syringe’s open-end by inserting the plunger.
- Position the plunger at the 30 ml reading on the syringe.
- Now, close the syringe’s tip and press the plunger to the maximum possible level. Release the plunger and then pull it to the open end of the syringe.
Observe the difference when the balloon filled with water is within a closed system of the syringe.
We observe in both cases, that when the pressure increases the volume decreases. Similarly, the volume increases when the pressure decreases.

